Abstract
Background With up to 50% of patients suffering from Non-Hodgkin`s lymphoma being refractory to or relapsing (r/r NHL) after treatment (M. Crump, et al. Blood 2017), this remains an unmet medical need. Glofitamab, a T-cell engaging bispecific antibody, has demonstrated significant single agent activity by augmenting T-cell activation and tumor infiltration in r/r NHL patients (ORR 53.7% in aggressive NHL and 81.3% in indolent NHL; M. Dickinson, et al. Blood 2021; A. Broeske, et al. Blood Advances 2022).
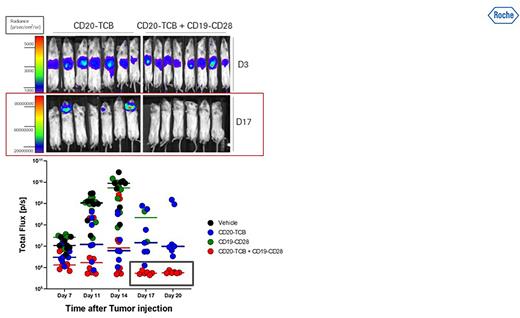
RO7443904 is a human bispecific antibody-like fusion protein that simultaneously binds to CD19 on B cells (i.e., tumor cells) and CD28 on T-cells. RO7443904 contains a monovalent low affinity CD28 binder and a silent FC domain that allows safe CD28 agonism without inducing autonomous T-cell activation, and therefore has no single agent activity. The expected mode of action (MoA) is to deliver a costimulatory signal 2 upon glofitamab-induced T-cell activation (i.e. signal 1) that further enhances T-cell effector function, prevents T-cell anergy and apoptosis, and induces a deep and durable immune response. In pre-clinical in vivo models (Figure 1) RO7443904 boosts anti-tumor activity of glofitamab without exhibiting any superagonistic activity and cytokine release.
Methods RO7443904 is being developed in combination with glofitamab in a phase I, open-label, dose-escalation study BP43131 (NCT05219513). The study is designed to evaluate safety, tolerability, pharmacokinetic (PK), and pharmacodynamic (PD) profile of escalating doses of RO7443904 in combination with glofitamab and its recommended phase 2 dose in participants with r/r NHL. Patients with r/r NHL, ECOG 0-1, with at least 2 prior systemic therapies, meeting standard organ function criteria and with adequate blood counts will be eligible. The dose escalation stage is divided into three parts (1a,b; 2; 3) followed by an expansion phase (part 4). The unique dose escalation design allows early adoption of subcutaneous (SC) administration of RO7443904 by introducing a single SC administration in cycle 4 (part 1b) followed by either a full SC administration from the first RO7443904 dose (part 3) or a SC administration from the second RO7443904 dose onwards (part 2).
Across the different study parts, the treatment schedule is consistent with a single fixed dose of obinutuzumab (1000mg intravenously) given at least 3 days prior to glofitamab step up dosing (2.5/10/30mg). RO7443904 is administered on cycle 2 day 8, one week after completion of the glofitamab step-up dosing. From cycle 3 day 1 onwards both drugs are administered concomitantly in a q3w schedule.
The full dose-escalation will be guided by a mCRM-EWOC design (modified Continual Reassessment Method, Escalation With Overdose Control). Patients will initially be recruited into the part 1a of the study in multiple participant cohorts, the starting dose for the first cohort is 150 μg (flat dose) and all administrations of RO7443904 are given intravenously. After reaching a minimum dose of 1 mg, Part 1b will start, where a single SC administration is introduced, with a potential to extend the SC administration to multiple cycles later on (therefore opening part 2 and/or part 3). Commencement of part 4, the expansion phase, including decision on the RO7443904 dose will be guided by a concerted review of safety, PK, and PD data from all dose escalation parts. The maximum duration of the study for each participant will be up to 18 months.
Tumor biopsies and peripheral blood biomarker analyses will be used to demonstrate MoA and proof of concept for an off-the-shelf flexible combination option providing signals 1 and 2.
Figure 1: Efficacy study in a disseminated DLBCL model in humanized NSG mice treated with monotherapy of glofitmab (0.15 mg/kg) or CD19-CD28 (1 mg/kg) as well as with a combination of both. The data suggest a strong anti-tumor effect when both agents are combined.
Disclosures
Dickinson:Roche, BMS, Novartis, Kite, Gilead, NKARTA, AdiCet Bio, Interius, Janssen, MSD, Amgen: Honoraria; Roche, Novartis, Kite, Gilead, MSD, Takeda, Celgene: Research Funding; Roche, BMS, Novartis, Kite, Gilead, NKARTA, AdiCet Bio, Interius, Janssen, MSD: Consultancy. Gritti:Roche, Takeda, IQVIA, Kite-Gilead, Italfarmaco, Ideogen, Genmab: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Scientific Consultancy; Roche, Takeda, Clinigen, Beigene, Incyte, Ideogen: Other: Training Activity; Roche, Sandoz: Other: Support for attending meetings. Carlo-Stella:AstraZeneca: Honoraria; Sanofi: Other: Consultancy/Advisory, Research Funding; Bristol Myers Squibb: Honoraria; Merck Sharp & Dohme: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Incyte: Honoraria; Janssen Oncology: Honoraria; Celgene/Bristol Myers Squibb: Other: Consultancy/Advisory; Roche: Other: Consultancy/Advisory, Research Funding; ADC Therapeutics: Honoraria, Other: Consultancy/Advisory, Research Funding; Karyopharm Therapeutics: Other: Consultancy/Advisory; Scenic Biotech: Other: Consultancy/Advisory. Walter:Roche, Gilead, Lilly, Beigene: Consultancy, Honoraria, Research Funding. Carlile:Roche Products Ltd: Current Employment; AstraZeneca, Roche: Current equity holder in publicly-traded company; Roche: Patents & Royalties: Patent. Getzmann:Hoffmann la Roche: Current Employment. Curdt:Roche Diagnostics GmbH: Current Employment. Harrop:Roche Ltd.: Current Employment. Keelara:Roche Ltd.: Current Employment. Korfi:Roche: Current Employment, Current equity holder in publicly-traded company. Labatut:Hoffmann la Roche: Current Employment. Michielin:Hoffmann la Roche: Current Employment. Mycroft:Roche Ltd.: Current Employment. Moore:Roche Diagnostics GmbH: Current Employment; F.Hoffmann-La Roche: Current equity holder in publicly-traded company. Nutbrown:Roche Ltd.: Current Employment. Palldino:A4P Consulting Ltd.: Current Employment. Whayman:Roche Ltd.: Current Employment. Sam:Roche Glycart AG: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Weisser:Roche: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties; Roche Diagnostics GmbH: Current Employment. Lechner:Roche Diagnostics GmbH: Current Employment. Morschhauser:Miltenyi: Membership on an entity's Board of Directors or advisory committees; Janssen: Speakers Bureau; Genentech: Consultancy; Genmab: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Allogene therapeutics: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Hutchings:Genmab: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; AbbVie: Consultancy; Incyte: Research Funding; Janssen: Consultancy, Research Funding; AbbVie, Celgene, Genmab, Janssen, Roche, Takeda: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Novartis: Research Funding; Celgene, Genentech, Genmab, Incyte, Janssen, Novartis, Roche, Takeda: Research Funding; Roche: Consultancy, Research Funding; Takeda: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal